What is the difference between GEL and AGM batteries?
GEL and AGM batteries are Valve-regulated lead-acid (VRLA) recombinant technology batteries. Both GEL and AGM batteries are considered to be of a starved electrolyte (DRY CELL) design. Both are sealed and considered non-hazardous - nonspillable.
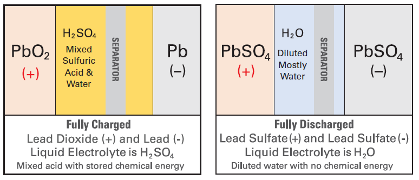
In an AGM or GEL battery, the electrolyte does not flow like a normal liquid.
In AGM batteries, all liquid electrolyte is trapped in a sponge-like mat of woven glass fibre separator material.
In a GEL battery, the electrolyte is mixed with a silicate additive which immobilizes (changes) the electrolyte into a GEL-like material consistency.
The “acid-starved” condition of GEL and AGM batteries protects the plates during heavy deep- discharges. The more acid-starved the batteries are, the more protection is given to the active materials. The Sulfuric acid in the battery electrolyte is fundamental to the discharge process. When the acid is depleted, the discharge process cannot go on.
Due to the physical properties of the GEL electrolyte and the batteries' inherently higher internal resistance, GEL battery power declines faster than an AGM battery’s as the temperature drops below 32°F (0°C).
AGM batteries excel for high current, high power applications and in extremely cold environments. GEL batteries tend to live longer in hot climates.
AGM batteries are better replacements for FLOODED batteries than GEL batteries when used in alternator-equipped applications. GEL batteries require lower (precisely controlled) charging voltages (mainly lower finishing voltages) than AGM or Flooded types. AGM battery charge voltages are more similar to Flooded charge voltages (14.4V – 14.7V) than GEL charge voltages (13.5V to 13.8V).
At room temperature, the difference between GEL and AGM batteries for higher current, high power applications is minimal.
